April 2024
Axolabs Berlin und Asahi Kasei Bioprocess, Teil der Asahi Kasei Gruppe, sind eine strategische Partnerschaft auf dem aufstrebenden Gebiet der Oligonukleotid-Therapeutika eingegangen. Die Partner werden zusammenarbeiten, um eine hochmoderne cGMP-Produktionsanlage für Oligonukleotide auf einer Fläche von 5.481 Quadratmetern in Berlin zu errichten. Ziel dieser Kooperation ist es, die Entwicklung und Vermarktung von Oligonukleotid-basierten Therapien zu beschleunigen, um die Lebensqualität von Patienten weltweit zu verbessern.
Für mehr Informationen : Pressemitteilung

Februar 2024
In Rahmen des „Tag der Unternehmen 2024“ hatte Axolabs Kulmbach Besuch von Schülerinnen und Schülern der 9. und 10. Klasse des Caspar-Vischer-Gymnasiums.
Der Tag bildet einen wichtigen Baustein des Moduls der beruflichen Orientierung und soll den Schülerinnen und Schülern einen ersten Einblick in die regionale Unternehmenswelt in Kulmbach geben. Das ganze Axolabs-Team hat sich über euren Besuch gefreut! Danke dass ihr da wart!

Dezember 2023
Das ist die Chance sich über Axolabs als Arbeitgeber zu informieren. Hier erfährt man alles über unsere Firma und unser Geschäftsfeld. Einfach vorbeikommen und informieren. Wir freuen uns schon jetzt auf jeden Besucher!

November 2023
Diese Woche hatte Axolabs Kulmbach das Vergnügen Dr. Claudia Höbartner zu begrüßen. Dr. Höbartner ist Professorin für Organische Chemie am Institut für Organische Chemie an der Universität in Würzburg. Unser Team durfte an Dr. Höbartners Seminar zum Thema "Emerging strategies for RNA modifications and labeling" teilnehmen und ganz Axolabs möchte ihr für ihre Beiträge zur Nukleinsäureforschung danken, welche Sie in der Vergangenheit geleistet hat und sicherlich auch in Zukunft leisten wird! Vielen Dank!

September 2023
Einen sensationellen 3. Platz belegte das „Axoletics-Lauf-Team“ von Axolabs Kulmbach beim diesjährigen Kessellauf. Das Team bestehend aus Verena Klaus, Fabian Frank und Ralf Stöcker bestritt hierbei eine Strecke von 14,3km mit 300 Höhenmetern und freute sich am Ende als sie ihren Preis entgegennehmen durften! Glückwünsche an das ganze Team!

Juli 2023
Im Juli hatte Axolabs Kulmbach Besuch des Oberbügermeisters der Stadt Kulmbach, Ingo Lehmann. Bei einem Rundgang durch unsere Räumlichkeiten und Labore konnte er sich selbst ein aktuelles Bild von Axolabs machen. Er war begeistert und sagte selbst: „Es ist beeindruckend, welche innovative Kraft von Axolabs ausgeht und dass die Grundlagenforschung für neue Arzneimittel vorangetrieben wird, ist eine gute Nachricht. Kulmbach entwickelt sich als Wissenschaftsstandort kontinuierlich weiter.“ Axolabs sagt „Danke“ für diesen erneuten Besuch unseres Standortes.

Juli 2023
Letzte Woche hatte Axolabs Kulmbach Besuch von den Schülern der Berufsfachschule „Medizinische Technologie für Laboratoriumsanalytik des Krankenhauszweckverbandes Bayreuth“. Bei einem Rundgang durch unsere Firma und deren Labore konnten die Schüler einen Einblick in die Arbeitswelt bei Axolabs erhalten und vielleicht startet der ein oder andere Schüler oder Schülerin sein Berufsleben bei uns! Vielen Dank dass ihr bei uns wart!

April 2023
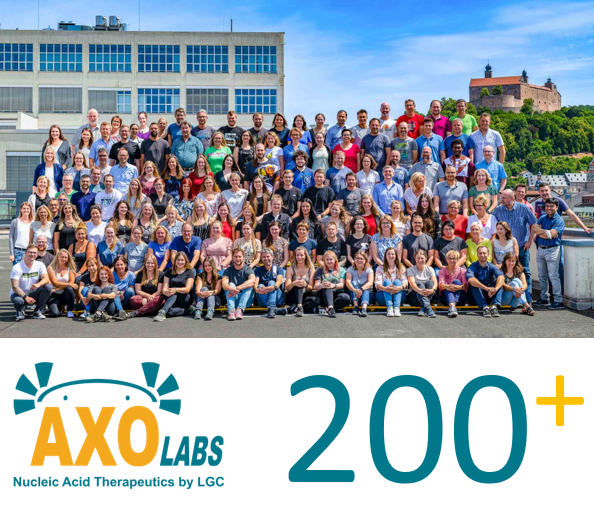
Wir freuen uns sehr, unseren 200. Mitarbeiter am Standort Kulmbach begrüßen zu dürfen!
Unser starkes Wachstum spiegelt die Stärke des Axolabs-Teams wider und erweitert unser Fachwissen, um Ihre therapeutischen Nukleinsäureprojekte weiter zu unterstützen.
January 2023
At Axolabs Kulmbach we are happy to announce that we are now an officially certified Service Provider of Oxford Nanopore Technologies long-read sequencing technique.
Quite recently Nature Methods has choosen the long-read sequencing technique as their "Method of the year".
Learn more: Nature Methods

November 2022
Our three locations (Berlin & Kulmbach, Germany & Petaluma, US) support customers across all stages of oligonucleotide development by harnessing the expertise of more than 20 years of experience in Nucleic Acid Therapeutics.
Our updated logo reflects a common vision for all locations; we aim to be the world leading and trusted service partner for delivering custom research and manufacturing.

June 2022
March 2022
On March 31, 2022, Hans-Peter Vornlocher stepped down from the day-to-day management of Axolabs taking the first step towards a well-earned retirement. His successor will be Philipp Hadwiger, Senior Director of Chemistry, an experienced colleague who has held leading positions at Axolabs (and its predecessor companies) for more than 20 years, and has played a key role in Axolabs’ growth and expansion. In anticipation of this change, Philipp has already been involved in the Axolabs’ extended management board in recent years.
Hans-Peter will continue to work part-time for Axolabs, advising our customers and driving the development of new technologies as Axolabs’ new Chief Scientific Officer (CSO). His enormous wealth of knowledge and experience will therefore still be available to our present and future customers.
With this structure, we can ensure a smooth management transition and continue to be a competent partner and service provider for our customers as we always have been, serving our customers individually and delivering projects with the highest quality.
Philipp will manage our rapidly and steadily growing company with his incumbent managing director colleagues, Dr. Ingo Röhl and Friedrich Sanders, all of whom report to LGC’s management board.
Kulmbach, 18 February 2022
Today Axolabs announced the third successful re-certification of its GLP bioanalytical testing facility. The German authorities formally certified Axolabs’ compliance with international Good Laboratory Practice (GLP), without any objections, as part of a recent audit of Axolabs' bioanalytical GLP facility. The authorities also certified Axolabs' GLP laboratories for the new testing category 1 (physical and chemical testing).
This significantly expands Axolabs' ability to support its customers with test item analysis (e.g. Dose Formulation Analysis) during GLP toxicology studies.
Ingo Röhl, Managing Director at Axolabs, said, “The successful GLP certification of the new test category provides an additional platform for even stronger collaboration with our clients in their oligonucleotide and mRNA therapeutic development activities.
Axolabs has been on a strong growth course since its foundation and is currently expanding its bioanalytical facilities and capabilities even further.
November 2021
Kulmbach, 29 September 2021
Today Axolabs announced the achievement of a major milestone at its site in Kulmbach, Germany. German authorities recently inspected Axolabs’ existing 400 m² GMP-CMC analytical facility and formally certified its compliance with international Good Manufacturing Practice (GMP) standards. Axolabs is now a certified test site for stability testing and the official release of human medicinal products and human investigational medicinal products, in particular therapeutics based on oligonucleotides and mRNAs, and including mRNA vaccines. This significantly expands Axolabs' capabilities in development solutions for therapeutic oligonucleotides and mRNAs.
Ingo Röhl, Managing Director at Axolabs, said, “The successful GMP certification provides an additional platform for an even stronger collaboration with our clients in their oligonucleotide and mRNA therapeutics development activities. We are glad we can add analytical services under GMP as a new dimension to our services in biology, synthetic chemistry and other analytical areas.”
The new capability in Germany complements LGC's established CMC analysis laboratories in the UK, as well as its cGMP manufacturing and oligonucleotide CMC laboratories in Petaluma, California. LGC can now offer its customers a global platform to support their drug development and manufacturing programs.
Axolabs has been on a strong growth course since its foundation and is currently expanding its Kulmbach facilities in all business areas.
Kulmbach - London, 1 October, 2018
Today, LGC Axolabs announces a significant investment at its site at Kulmbach, Germany, to expand the capacity of its specialist therapeutic oligonucleotide development solutions business and create a new GMP analytical capability.
Due to open in February 2019, the new 800m² labs comprise additional bioanalysis and synthetic chemistry labs as well as a new GMP CMC analytical capability for batch release testing of oligonucleotide-based drug substances and drug products in the EU.
The new facility will complement LGC’s established bioanalytical and CMC analytical labs in the UK, as well as their oligonucleotide cGMP manufacture and CMC labs in Petaluma, California which is due to run its first cGMP manufacturing campaigns later this year. LGC now offers its clients a truly global platform to support drug development and manufacturing.
Hans-Peter Vornlocher, Managing Director, Research at LGC Axolabs, said, “This investment provides a platform for growth of our oligonucleotide therapeutics development activities, expanding our total site capacity to around 4,500m2 and to around 100 scientists. We are also glad to be able to add GMP analytical services as a new dimension to our biology, synthetic chemistry and other analytical services”.
David Griffiths, Managing Director, Pharma & Health Solutions, LGC, said, “We’re excited about this expansion which not only supports growth of our Kulmbach site but is also part of a larger international growth strategy for LGC’s drug development solutions business giving access and greater flexibility for our clients”. The investment in Kulmbach supplements LGC’s announcement last year of further investment at its Petaluma site as well as the recent completion of the first phase in a long-term major investment to expand capacity of its bioanalytical labs in Fordham, UK.